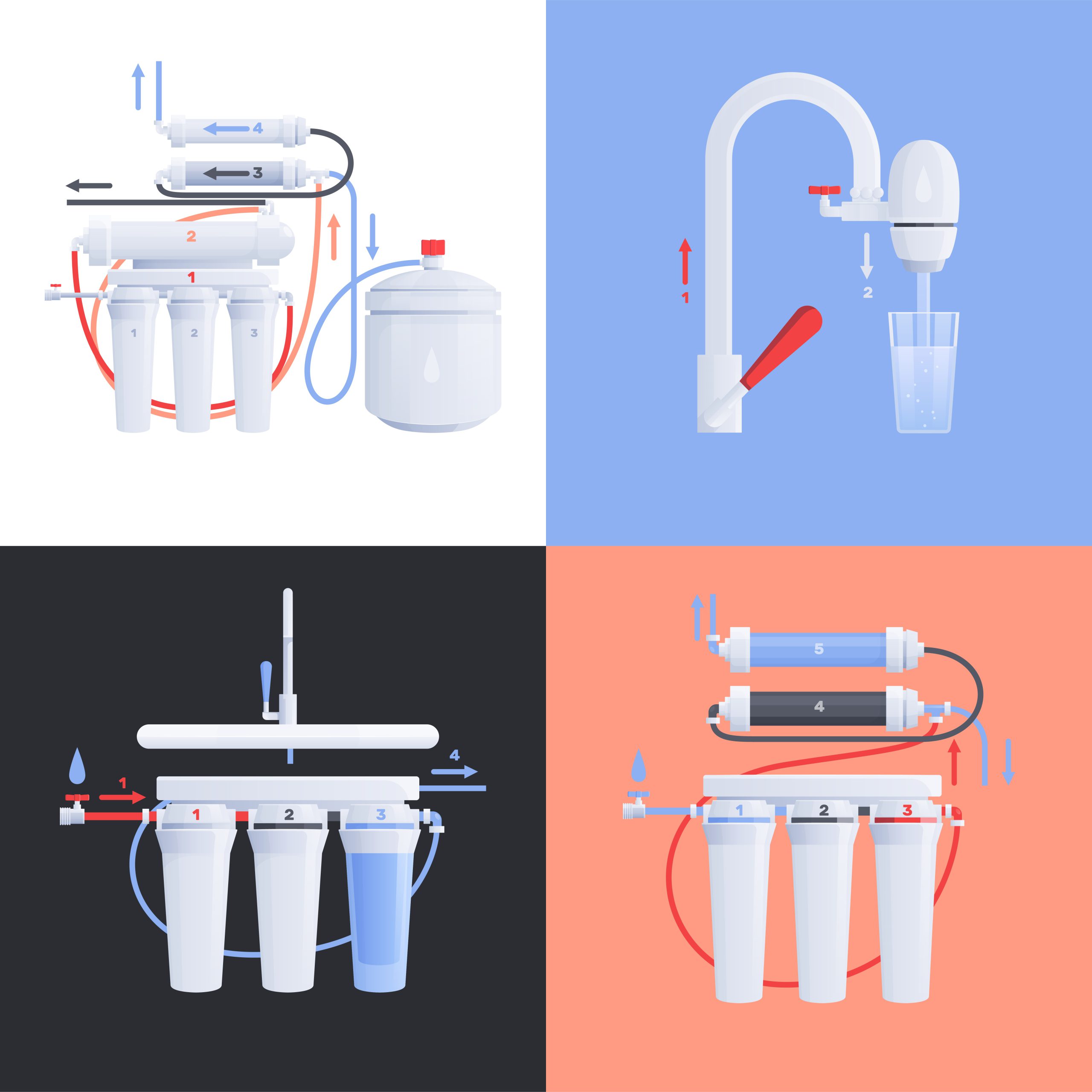
The importance of using anti-scale in the RO plant
What is the process of calcification or sedimentation?
The term anti-scale is the reduction of sludge formation or in technical terms – anti-scaling, in industrial equipment such as reverse osmosis (RO) systems, many manufacturers have heard of. Scaling means the deposition of inorganic or mineral fouling particles on the surface of the membranes, which leads to clogging of the membrane pores. The deposition process is the deposition of low-soluble salts on the surface of the membrane. Calcification is the accumulation of suspended solids and microorganisms on the surface of the membrane. Calcification in reverse osmosis systems is a normal phenomenon. It can be controlled through filtering, appropriate filtration, and chemical washing when needed.
What is chemical scaling?
Chemical scaling caused by solution mineral compounds in water such as sodium carbonate, calcium sulfate, silica, silicon dioxide, and barium sulfate, can reduce the efficiency of membrane filtration and membrane filter systems, increase pressure and thus increase energy consumption and associated costs, and reduce quality water, and reduces frequent washing and malfunctions. It is worth noting that the useful life of the membrane filter may be reduced due to the phenomenon of chemical scaling.
Precipitate formation begins when the salt concentration exceeds its solubility under solution conditions. Primary crystal cores play a catalytic role in the formation of larger salt crystals. These salt crystals come out of the suspended state and begin to precipitate as long as they reach the required size and mass. The sedimentation process will continue until the ions that make up the salt are saturated in the solution.
The reason for the formation of deposits on the membrane filter:
There are several reasons that lead to the formation of sediments on the surface of the reverse osmosis membrane, the most important of which are:
- Increasing the concentration of minerals of the solution in the water entering the membranes
- PH increase
- Creation and augmentation of the surfaces required for deposition (which will be multiplied by the continuous chemical scaling of mineral deposits to this desired level)
- Water temperature increase
What is anti-scale?
The anti-scale is used as an additive in the primary treatment process in reverse osmosis systems. Anti-scale is used as an active ingredient for sedimentation control in reverse osmosis membranes. Before transferring the water to reverse osmosis, an antiscalant with the appropriate concentration is injected into the water feed flow. The chemicals used in the composition of antiscalants prevent the formation of mineral deposits on the surface of the membranes. Injection of appropriate concentrations of antiscalants leads to controlling the formation of types of mineral deposits such as calcium carbonate, calcium sulfate, iron deposits, and others on the surface of the membrane.
Importance of using an anti-scale?
The use of anti-scale in reverse osmosis systems may reduce costs related to the repair and maintenance of water purification systems. By continuously injecting anti-scale to the reverse osmosis system, the scaling current will decrease rapidly. The amount of scale inhibitor used as a descaling agent depends on the concentration of soluble salts in the feedwater or the place of application.
Due to the presence of inorganic and mineral salts of the incoming water solution to the reverse osmosis water treatment system and water desalination machine, blockage and deposition in the polyamide or cellulose micropores of the reverse osmosis membranes, the phenomenon of calcification occurs. Precipitates on polyamide films include calcium carbonate, calcium sulfate, barium sulfate, and strontium sulfate. Silica and calcium fluoride deposits are less common but are a problem. To prevent these soluble salts on the membrane, they are injected into the water entering the anti-hull reverse osmosis system.
Disadvantages of using substandard antiscalants in reverse osmosis systems:
- Reduces production capacity in water purification systems
- Increases the number of water-soluble minerals
- Increases pressure on floor pumps and reduce pump life
- Energy consumption during the reverse osmosis process increases
- Reducing membrane life and early replacement
Mechanism of action of anti-scale
The mechanism of action of antiscalants is the disruption of the mineral deposit production reactions, or in other words, this substance by some substances in its structure can reduce the rate of reaction of the alkali compound with all mineral ions in the water, in this case during the passage of water through the polyamide membrane. The possibility of sediment formation is eliminated.
Calcium carbonate, calcium sulfate, and strontium sulfate are among the minerals that deposit on the membrane.
In general, all systems in which membrane technology is used for water treatment are subject to membrane pore blockage, and the most important types are reverse osmosis desalination systems.
Amount of anti-scale injection
The amount of antiscalant injected depends on various parameters such as water analysis, flow rate, temperature, pH, system recovery rate, water source, membrane type, and how the membranes are arranged. The rate of injection of antiscalants ranges from 1 to 6 ppm.
Problems arising from not using anti-scale:
The problems that occur mostly due to chemical scaling in membrane filters are the following:
- Decreased flow quality and quantity of flowing water
- Increased low pressure, required pressure, energy consumption, and cost.
- Frequent washing with fewer intervals to optimize processes that reduce the useful life of membranes and increase costs
- Frequent and long-term malfunctions
- Membrane dispersion upon collision with mineral deposition crystals
- One of the useful ways to prevent the occurrence of the problems mentioned is the use of anti-scale.
Poor quality anti-scale increases the need to wash the membrane filter which is not good at all for water purifiers. In addition, the poor material causes the membrane filter to be replaced quickly, which leads to a shortening of the useful life of the filter and, shortly after, causes total damage to the device. The useful life of the pumps is also affected by this inferior product. More importantly, this ideal material, if it is not of good quality, is also harmful to the water and reduces the yield and quality of pure water. As a result, low-quality antiscalants cause many problems, and on the contrary, the higher the quality of the anti-fouling materials, the higher the quality of the treated water.
Sedimentation and blockage of the films lead to a decrease in the effective area between the materials and it reduces the turbulence of the flow and may result in a polarization of the focus on the surface of the membrane. The higher the concentration of the solution on the surface of the membrane, the greater the percentage of solute it will pass through. When the effective membrane level decreases, the turbulence will decrease and the surface cleaning process of the membrane will be difficult because the possibility of sending the chemical solution to the surfaces blocked by solid materials will be reduced. It is possible to prevent precipitation by using the correct efficiency of the system and the injection of chemicals.
Method of performance of antiscalant or descaling agent
Sedimentation inhibitors or anti-scale slow down the sedimentation process by preventing the growth of mineral salt crystals. These substances form on the surface of the salt crystal and adsorb on the surface of the polyamide film. By preventing further adsorption of supersaturated salts on the crystal surfaces, they reduce the rate of expansion of the salt crystals, so that the primary crystal cores do not reach a sufficient size or concentration for precipitation. Most inhibitors have dispersal properties. The properties of suspension or dispersion occur by trapping suspended particles of salt, iron, or solid organic matter and causing them to be repelled by other ions in the solution. This process prevents particles from accumulating and forming larger particles that can precipitate. Antiscalants mainly lead to the effective removal of sediments from reverse osmosis membranes through several mechanisms:
1 . Threshold inhibition: the ability to combat sedimentation to preserve water-soluble mineral solutions
2 . Crystalline variation: Anti-scalants change the crystalline structure and change the crystal structure in the form of non-sticky deposits, causing a decrease in calcification of the deposits on the surface of the reverse osmosis membrane. As the crystal precipitates grow, the negatively charged antiscalants absorb the positive crystal ions and, by upsetting the charge balance required for crystal growth, prevent the deposition of positive crystals on the surface of the membranes.
3 . Dispersant: Antiscalants are primarily anionic in structure and the proper positioning between the colloidal and cationic crystalline particles causes the particles to disperse in the feed water.
Sedimentation inhibitors are only effective in reducing sedimentation formation or decreasing the process and swelling of sediment particles and cannot stop the sedimentation process completely. However, in a reverse osmosis system, it is sufficient for the anti-scale to work well from scale formation to the exit of the concentrated current from the system.
How to determine the probability of water calcification
Determining the potential for water calcification is complex and is usually done by related programming. This software is mostly produced by companies producing antiscalants. In these programs, the required amount of scaling inhibitor is calculated by the large-scale probability of calc formation in the discharge current.
One of the most important points in seawater treatment is that the probability of the presence of boron (bor) in this type of feed water, is high and must be separated at a higher pH (in the range of 9-11). much. Since the pH should not change much, adding acid is not at all helpful and the only solution would be an anti-scale injection. Most antiscalants are oxidized by strong oxidants such as chlorine and bromine, and not only lose their properties but also cause biological contamination by destroying the molecular structure. Therefore, these oxidizers must be removed in the pretreatment system prior to the injection site (eg a carbon filter at the beginning of the line that absorbs chlorine).
The chemical structure of the anti-scale molecule cannot pass through the membrane. Therefore, no concentrations of this solution enter into the water produced by reverse osmosis. But in terms of the environmental effects, it is necessary to check the exit of the solution from the discharge stream. In this context, the amount of injection must be controlled for the line and with the need to be injected, the lowest possible percentage of injection is injected.
Sea water antiscalant
Antiscalants play a major role in seawater desalination. Due to water scarcity in recent years, water desalination has become of great importance. One such solution is the use of reverse osmosis devices by which seawater is desalinated. Water entering the reverse osmosis system contains soluble salts of calcium, magnesium, and other minerals. Seawater antiscalants are mainly based on polymers.
The use of Reverse Osmosis Anti-scale for a water care company includes the following advantages:
The special application of RO systems with high sediment retention efficiency
The superior performance of anti-scale in comparison with domestic and foreign competitors
Easy to use with accurate and continuous sediment inhibiting performance
Reasonable price compared to local competitors
High efficiency of sediment inhibition against types of carbonate and sulfate deposits
Using on all cellulose and polyamide films
You can order anti-scale from here.